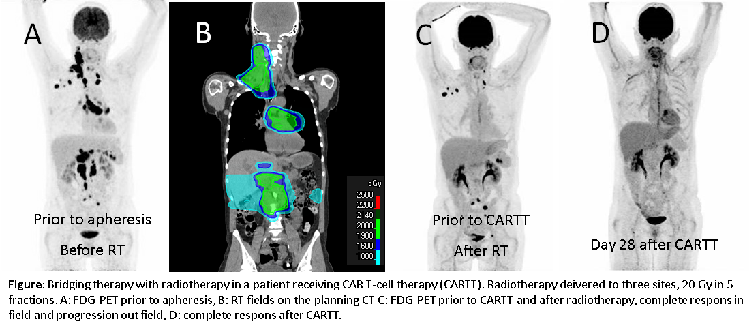
Radiotherapy as bridging strategy in Large B-cell lymphoma patients selected for CAR T-cell therapy
OC-0206
Abstract
Radiotherapy as bridging strategy in Large B-cell lymphoma patients selected for CAR T-cell therapy
Authors: Anne Niezink1, Max Beijert2, Jaap van Doesum3, Christina T. Muijs2, Johannes A. Langendijk2, Dianne M. Busz2, Anne P.G. Crijns2, Tom van Meerten3
1University Medical Center Groningen / University of Groningen , Radiation Oncology, Groningen, The Netherlands; 2University Medical Center Groningen / University of Groningen, Radiation Oncology, Groningen, The Netherlands; 3University Medical Center Groningen / University of Groningen, Hematology, Groningen, The Netherlands
Show Affiliations
Hide Affiliations
Purpose or Objective
Patients with Large
B-cell lymphoma (LBCL) who have refractory or relapse disease
after two lines of systemic therapy (ST) have a very poor prognosis. However, for
these patients anti-CD19 chimeric antigen receptor T-cell (CART) therapy has
emerged as a potential curative treatment regime as approximately half of the
patients achieve long-term disease-free survival. CAR T-cells are generated
from autologous T-cells, collected through an apheresis procedure. The logistics and
production are time consuming and may take up to 4-6 weeks.
Many patients in
which CART therapy (CARTT) is indicated have symptomatic and progressive disease
during the production period. Bridging therapy, referred to therapy
administered after apheresis until CART infusion may be indicated. Early reports
on radiotherapy (RT) as a bridging strategy have shown feasibility, safety and effectivity, but patient numbers
were limited.
Here we present our
experience with bridging in patients selected for CART therapy in a relatively
large cohort. The current analysis focuses on the evaluation of the response
rates.
Material and Methods
All patients treated
with CARTT are included in a prospective data registration program including
data on patient and tumor characteristics, treatment, toxicity and outcomes.
For this analysis all patients with LBCL who underwent apheresis for CARTT were
included. Bridging therapy may consist of steroids, chemotherapy, RT or
a combination of these treatments. Responses to bridging
therapy were based on 18F-Fluordeoxyglucose PET (FDG-PET) before CARTT
and survival outcomes are reported.
Results
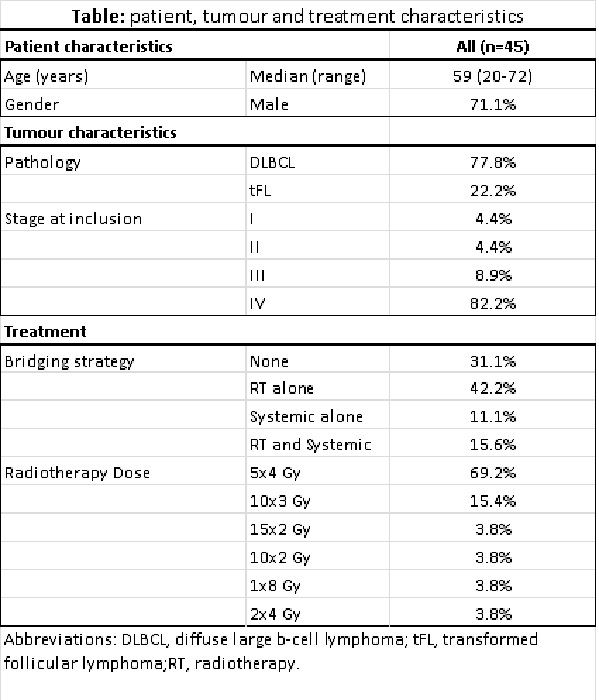
In total 45 patients
underwent an apheresis procedure. Fourteen of these patients (31.1%) did not receive
bridging treatment, 19 (41.2%) underwent RT alone, 5 (11.1%) received ST alone (steroids
or chemotherapy) and 7 (15.6%) received ST and RT (See table). RT was given on bulky tumor
or burdensome lesions, in the majority of patients to a total dose of 20 Gy in
5 fractions (See figure). Eighty-one percent of patients had an infield response to RT. All
patients who received RT alone had out of field progression, compared to 87% in
patients who received RT and ST and 66% in patients after ST alone.
Finally 41 patients
(91.1%) received CARTT, while 3 patients did not due to rapid progression and
no residual disease (1). The 1-year overall survival of patients who did not receive
bridging treatment was 90%, compared to 74.1% in patients bridged with RT alone
and 46.7% in patients treated with ST or combined treatment. 1-year progression
free survival was 62.3% versus 48.9% and 40.0%, respectively.
Conclusion
Bridging the time between apheresis and infusion is
a critical phase in CARTT. Selection of bridging treatment type is based on
prior treatment, tumor load and symptoms. RT is a good alternative for ST in
this heavily pre-treated patient population with a control rate of 81%. Given
the high progression rate, close follow-up is needed during the bridging phase.