A national framework for the rapid translation of novel radiotherapy-drug combination strategies from the laboratory to the clinic: the Cancer Research UK RadNet radiotherapy-drug combinations working group - PDF Version
Anthony Chalmers1,2, Lauren Hewitt3,4, Kaye Williams5,6
1School of Cancer Sciences, University of Glasgow, UK
2Director, Cancer Research UK RadNet Centre Glasgow, UK
3Division of Cancer Sciences, University of Manchester, UK
4Project Manager, Cancer Research UK RadNet Centre Manchester, UK
5Division of Pharmacy and Optometry, University of Manchester, UK
6Deputy Director, Cancer Research UK RadNet Centre Manchester, UK
Despite decades of laboratory research and many promising publications, very few novel radiotherapy-drug combinations have advanced to the clinic and actually improved outcomes for cancer patients. While many articles have been written describing the bottlenecks and roadblocks that contribute to this situation [1,2], there have been relatively few systematic or sustained attempts to improve it. In 2019, after several years of lobbying from the radiation research community, Cancer Research UK (CRUK) made an unprecedented investment in radiotherapy research through the establishment of seven UK ‘centres of excellence’ that together form the CRUK RadNet network. This network nurtures research programmes in each centre and features working groups that extend across and beyond the individual centres.
Kaye Williams (Manchester, UK) and Anthony Chalmers (Glasgow, UK) recognised that this offered an opportunity to change the landscape of radiotherapy-drug combination research and established the radiotherapy-drug combinations working group. The aim of the group, which was established and ratified in 2020, is to support researchers and laboratories across the UK, to encourage and enable them to work with each other and with industrial partners to generate robust preclinical evidence to underpin high-quality clinical trials. With essential project management support from Lauren Hewitt (Manchester, UK), the working group has grown rapidly to encompass membership from all seven RadNet and additional UK centres and to build effective partnerships with a broad range of stakeholders that include CRUK’s commercial partnerships team, the National Cancer Research Institute’s radiotherapy group (previously known as CTRad) and multiple pharmaceutical and biotechnology companies.
There are four cornerstones of the working group:
(1) the people – a cohesive community of scientific and clinical researchers working together in multidisciplinary groups;
(2) the technology – all researchers have access to state-of-the-art, quality-assured laboratory radiation facilities;
(3) the models – a broad repertoire of preclinical models that cover the relevant tumour types and enable measurement of associated normal tissue toxicities; and
(4) the collaborations – easy access to industrial and academic partners, funding bodies, and other consortia across the UK and beyond.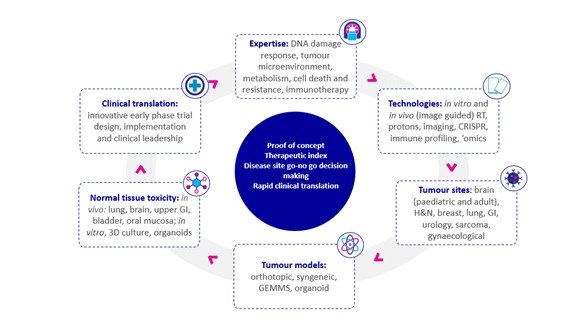
A key aim of the working group is to train and inspire the next generation of radiation researchers and to build capacity in this area, which has historically lacked critical mass. With this in mind, and to enable more detailed discussion and development of new ideas, we established five focus groups. Each of these is led by an early career researcher (some clinical, some non-clinical) and is focused on a distinct scientific theme: the DNA damage response, cell death and resistance, the tumour microenvironment, immunotherapy, and cancer metabolism. At the suggestion of our focus group leaders, we are developing a training workshop that we hope will equip these young researchers with the skills, contacts and knowledge necessary to translate promising research concepts into early-phase clinical trials. So far, the most popular components of the workshop have been the ‘real world exemplars’ of specific projects, both successful and unsuccessful.
There are promising signs that industry and biotech are interested in working with the working group to develop and refine new therapeutic approaches. Working group members have been approached by small and large companies as well as academic researchers, and potential new projects are discussed within the main group before they are allocated to focus groups for further development. A key feature of our approach is the identification of research labs that have complementary skills and models and offer a team-based approach to potential investors and partners. No single laboratory can provide all the necessary tumour- and normal-tissue models, but by working together a convincing package of preclinical data can be assembled that informs and supports subsequent clinical-trial design and delivery.

Kaye Williams

Anthony Chalmers

Lauren Hewitt
Sources
- Sharma RA, Plummer R, Stock JK, Greenhalgh TA, Ataman O, Kelly S, Clay R, Adams RA, Baird RD, Billingham L, Brown SR, Buckland S, Bulbeck H, Chalmers AJ, Clack G, Cranston AN, Damstrup L, Ferraldeschi R, Forster MD, Golec J, Hagan RM, Hall E, Hanauske AR, Harrington KJ, Haswell T, Hawkins MA, Illidge T, Jones H, Kennedy AS, McDonald F, Melcher T, O'Connor JP, Pollard JR, Saunders MP, Sebag-Montefiore D, Smitt M, Staffurth J, Stratford IJ, Wedge SR; National Cancer Research Institute CTRad Academia-Pharma Joint Working Group. Clinical development of new drug-radiotherapy combinations. Nat Rev Clin Oncol. 2016 Oct;13(10):627-42.
- Ahmad SS, Crittenden MR, Tran PT, Kluetz PG, Blumenthal GM, Bulbeck H, Baird RD, Williams KJ, Illidge T, Hahn SM, Lawrence TS, Spears PA, Walker AJ, Sharma RA. Clinical Development of Novel Drug-Radiotherapy Combinations. Clin Cancer Res. 2019 Mar 1;25(5):1455-1461.