PhiRO award
ESTRO 2025 Congress report
In the Netherlands, normal tissue complication probability (NTCP) models are used to guide decision-making in the treatment of head-and-neck cancer (HNC). However, current NTCP models, whether they are based on logistic regression, deep learning (DL), or other approaches, are designed to predict only a single radiation-induced toxicity. This approach overlooks the fact that patients undergoing radiotherapy are at risk of developing multiple, potentially interrelated toxicities. For instance, a patient who experiences dry mouth (xerostomia) or sticky saliva may also be more likely to suffer difficulties swallowing their food (dysphagia). The research that we presented at ESTRO 2025 showed how we can address this gap by developing and evaluating a multi-toxicity DL NTCP model that can predict multiple late toxicities in HNC patients simultaneously.
Our primary cohort consisted of 1,090 patients treated with (chemo)radiotherapy at the University Medical Centre Groningen (UMCG). This cohort was divided into development (80%) and testing (20%) sets. We modelled five toxicity endpoints: aspiration, dysphagia, sticky saliva, taste alteration, and xerostomia, all six months after radiotherapy. Patient-reported outcomes were used for most endpoints, except for dysphagia, which was clinician-rated. We also observed that there were statistically significant correlations between these toxicity endpoints within our cohort. We developed two types of DL models: (i) five independent single-toxicity models, each used for one of the above-listed toxicities; and (ii) a multi-toxicity model used to predict all five toxicities simultaneously. Input data included 3D distributions, CT images, organ-at-risk segmentations, and relevant clinical factors. Additionally, we compared the performance of these DL models with that of the logistic regression models that are currently used in clinical practice at UMCG.
The multi-toxicity model demonstrated superior predictive performance compared with the single-toxicity models on the independent test set for aspiration, dysphagia, and xerostomia. However, it showed slightly reduced performance for sticky saliva and taste alteration compared with both the single-toxicity models (logistic regression and DL). These results suggest that although the multi-toxicity model can capture inter-toxicity interactions, it may not universally outperform single-toxicity approaches for all endpoints.
Our presentation highlighted the potential of multi-toxicity modelling approaches to enhance NTCP model performance by accounting for interactions between toxicities. Although the multi-toxicity approach did not consistently outperform the single-toxicity models for all endpoints, it showed clear benefits for specific toxicities, underscoring the need for further exploration and optimisation. Our findings represent an important step towards enhanced decision-making in personalised treatment by demonstrating new approaches that can be used to improve the accuracy of NTCP predictions. Ultimately, such approaches can reduce late radiation-included toxicities in HNC survivors.

Daniel Connor MacRae
Department of Radiation Oncology, UMCG, the Netherlands
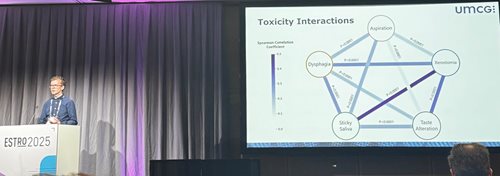

The multi-toxicity modelling project being presented in the ‘highlights predicting toxicities’ session at ESTRO 2025. Here, the correlations among the different toxicity endpoints in our patient cohort are being presented.