Best Physics Paper Award
ESTRO 2025 Congress Report
Proton therapy is a powerful tool for brain tumours, offering precise targeting that spares healthy tissue. But this precision brings a challenge: the biological effectiveness of protons can vary, especially as the particles slow down and stop.
Clinically, we assume protons are 10% more effective than photons, using a constant relative biological effectiveness (RBE) of 1.1. However, research suggests this may be too simplistic. As protons move through tissue, their energy deposition per path length, the linear energy transfer (LET), increases, enhancing their biological effect, particularly just beyond the tumour. Whether this variability in RBE leads to an increase in side effects remains a key question.
Late radiation-induced contrast-enhancing brain lesions (CEBL), frequently observed on follow-up MRI after radiotherapy, offer a unique model to study this. While often harmless, they can mimic tumour recurrence and trigger unnecessary or even harmful interventions. Predicting and preventing CEBL is, therefore, clinically important.
Previous studies identified three main risk factors for CEBL after proton therapy: dose, LET, and proximity to the brain’s ventricles. Including LET alongside dose improved model performance, supporting a variable proton RBE. In addition, the models pointed to a particularly radiosensitive region near the ventricles.
What we did
To explore these findings further, we analysed data from 156 brain tumour patients treated with proton or photon therapy. We tested existing proton-based prediction models [1,2] and, for the first time, developed new models for photon and combined proton–photon cohorts.
We built:
• Patient-level models to predict which patients might develop lesions
• Voxel-level models to predict where lesions are likely to occur
Key findings
- Validation of prior models: Earlier models performed well in our independent proton cohort, confirming dose, LET, and ventricular proximity as robust predictors, supporting a variable, LET-dependent RBE.
- Periventricular sensitivity in photon data: Lesion risk near the ventricles was also seen in the photon cohort, suggesting this sensitivity is not proton-specific but a broader radiobiological effect.
- Combined model insights: In the combined proton–photon dataset, the strongest predictor was a dose–volume metric calculated using a variable, LET-based RBE, rather than a fixed value, further reinforcing its clinical relevance.
Conclusions
1. The periventricular region is a radiosensitive area across radiation types.
2. There is clinical evidence that proton RBE varies with LET.
These insights argue for two changes in treatment planning: treating the periventricular region as an organ-at-risk and using a variable RBE instead of a constant value. This strategy is now being tested in the multicentre INDIGO trial, which evaluates LET-aware, model-guided planning to reduce CEBL risk [3,4].
Looking ahead
Does variable RBE also influence rarer but more severe effects, like optic toxicity? Answering this question will require larger, collaborative, multicentre studies.

Martina Palkowitsch
OncoRay – National Center for Radiation Research in Oncology, Faculty of Medicine and University Hospital Carl Gustav Carus, TUD Dresden University of Technology, Helmholtz-Zentrum Dresden-Rossendorf, Dresden, Germany
Email: martina.palkowitsch@oncoray.de
LinkedIn: www.linkedin.com/in/martina-palkowitsch
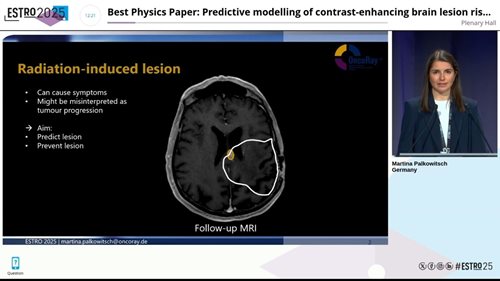
Follow-up MRI showing a radiation-induced brain lesion. Presented at ESTRO 2025.
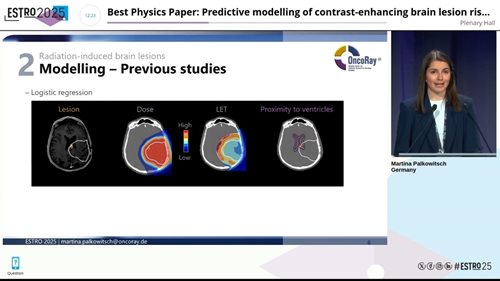
Predictive factors for radiation-induced brain lesions following proton therapy: dose, LET, and proximity to the ventricles. Presented at ESTRO 2025.

Martina Palkowitsch receiving the Best Physics Paper Award at ESTRO 2025, presented by Christian Richter, Chair of the ESTRO 2025 Physics Track.
References
[1] Bahn E, et al. Late contrast-enhancing brain lesions in proton-treated patients with low-grade glioma: clinical evidence for increased periventricular sensitivity and variable RBE. Int J Radiat Oncol Biol Phys 2020;107:571–8. https://doi.org/10.1016/j.ijrobp.2020.03.013 .
[2] Eulitz J, et al. Increased relative biological effectiveness and periventricular radiosensitivity in proton therapy of glioma patients. Radiother Oncol 2023; 178. https://doi.org/10.1016/j.radonc.2022.11.011.
[3] ClinicalTrials.gov Identifier: NCT05964569
[4] Sallem H et al. A model-based risk-minimizing proton treatment planning concept for brain injury prevention in low-grade glioma patients. Radiother Oncol 2024; 201:110579. https://doi.org/10.1016/j.radonc.2024.110579.